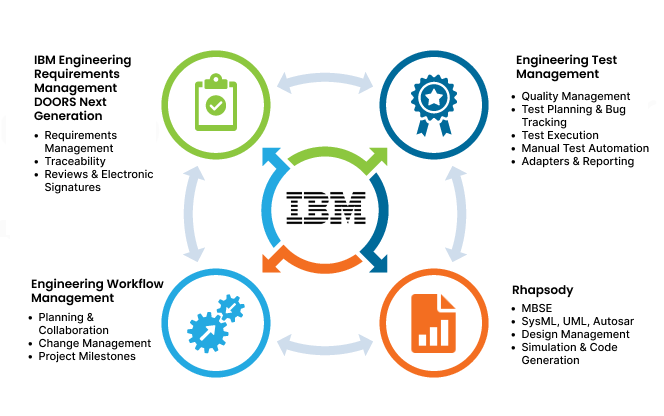
IEC 62304 Compliance with IBM ELM
Learn how IBM ELM helps you achieve compliance with the international standard IEC 62304 to satisfy all your workflow needs.
Overcoming the challenge of IEC 62304 Compliance
Software Development in the age of innovation and progress is causing market disruption and brings many growing concerns such as:
- Concerns over Cybersecurity
- Software becoming rapidly more Complex
- Requirements for Regulatory Approval
- Need for fast delivery, decision-making, and deployment
While still being able to contain all the costs, market release, and maintaining the quality at the same time. While the risk of failure is high, overcoming the challenge of IEC 62304 Compliance can help you succeed.
Overcoming the challenge of IEC 62304 Compliance
Software Development in the age of innovation and progress is causing market disruption and brings many growing concerns such as:
- Concerns over Cybersecurity
- Software becoming rapidly more Complex
- Requirements for Regulatory Approval
- Need for fast delivery, decision-making, and deployment
While still being able to contain all the costs, market release, and maintaining the quality at the same time. While the risk of failure is high, overcoming the challenge of IEC 62304 Compliance can help you succeed.

Testing
planning
Linked to requirements
Comprehensive test plan
Scope, Timeline, Resources
Risk assessment

Requirement driven testing
Test enviroments coverage
Manual test authoring
Test lab management

Execution
Manual test execution
Drive test automation tools
Record test results
Submit & Track defects

Status and progress tracking
Customizable live dashboard
Compliance and quality audit
Real-time metrics and reports

planning
Linked to requirements
Comprehensive test plan
Scope, Timeline, Resources
Risk assessment

Requirement driven testing
Test enviroments coverage
Manual test authoring
Test lab management

Execution
Manual test execution
Drive test automation tools
Record test results
Submit & Track defects

Status and progress tracking
Customizable live dashboard
Compliance and quality audit
Real-time metrics and reports
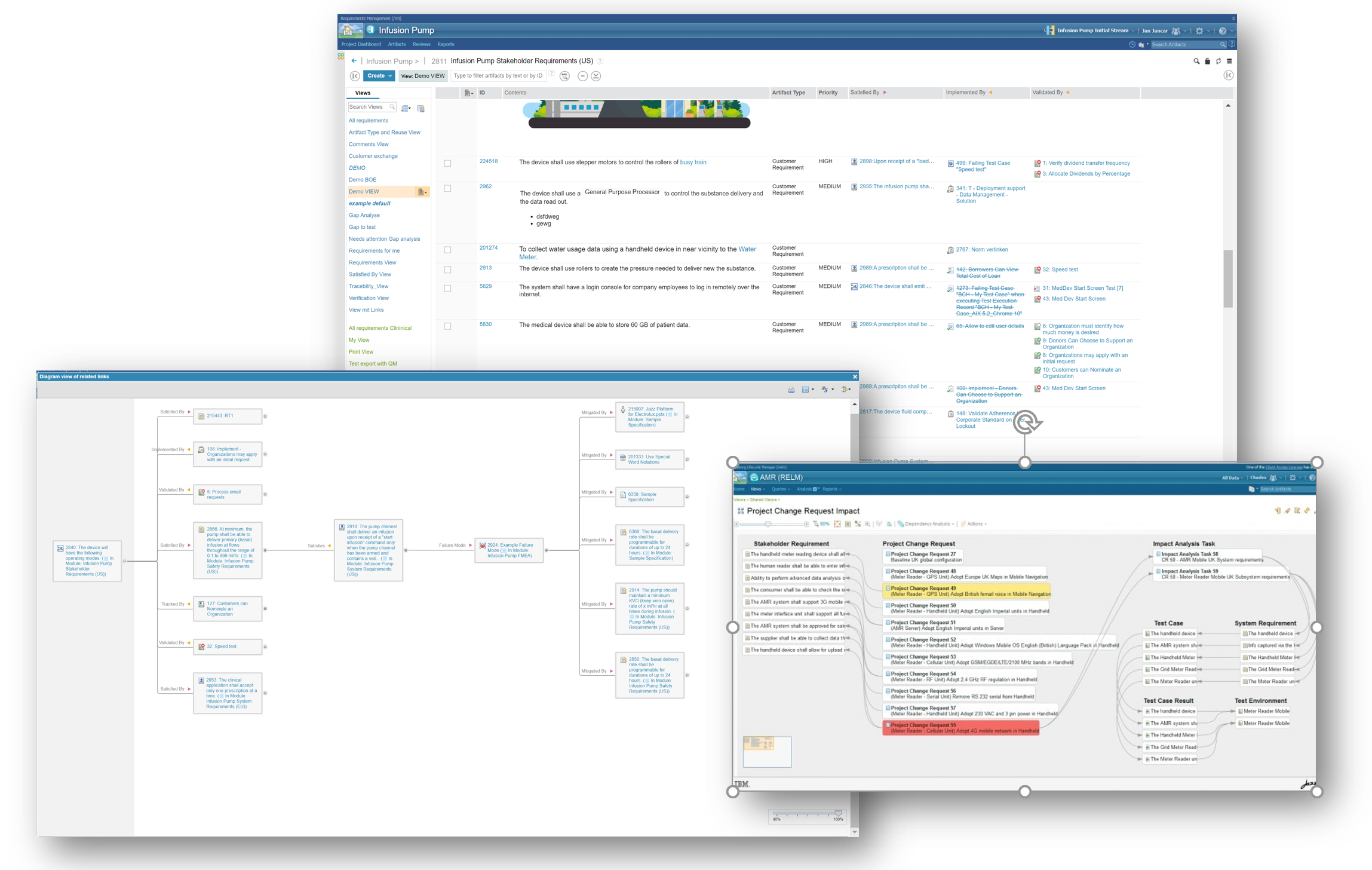
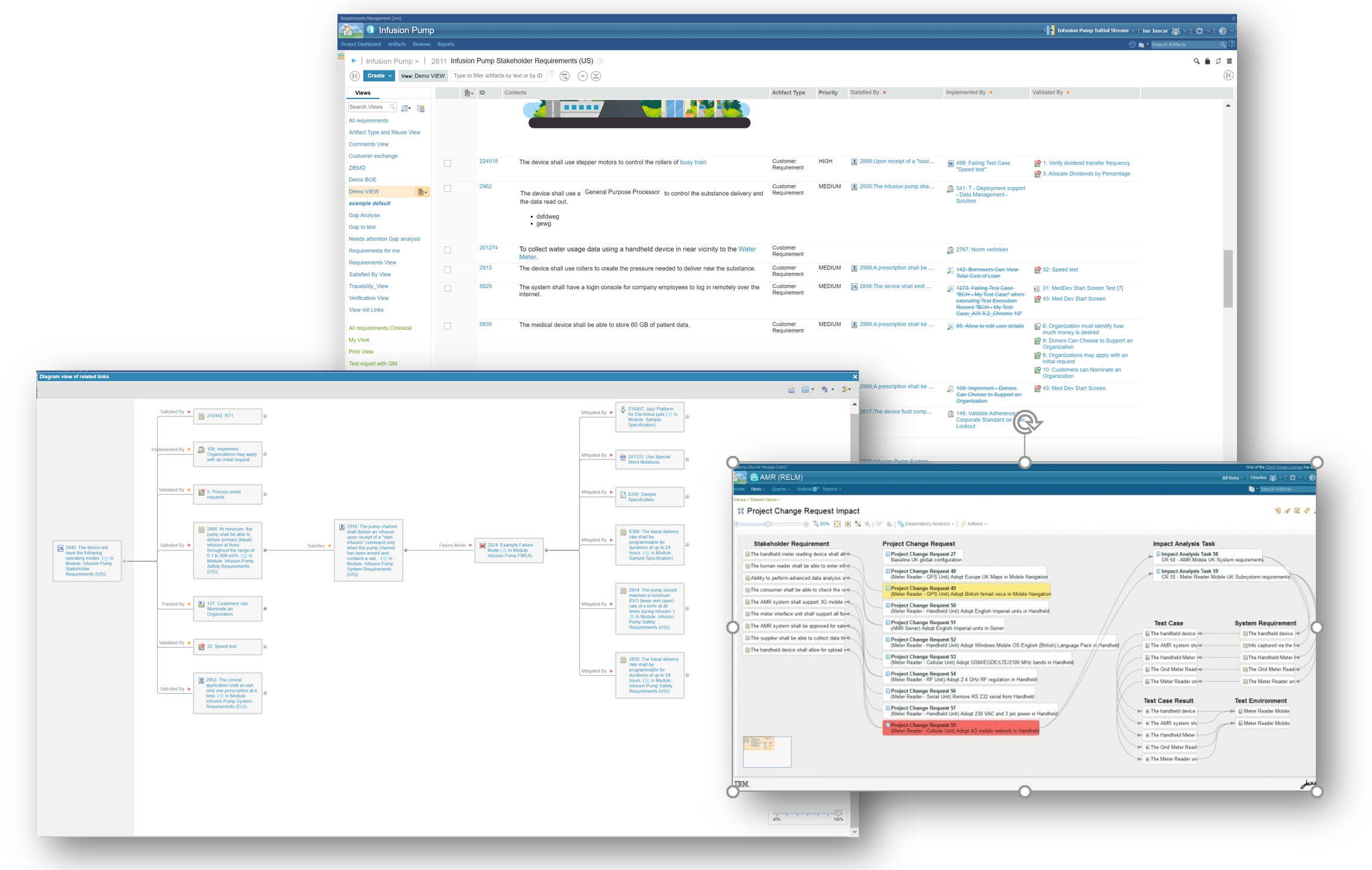
Traceability and Transparency
Achieve IEC 62304 compliance with Requirements traceability. IBM ELM offers great help with the support of Software Requirement Analysis by tracing software requirements to its parent requirements, while also linking to test cases and test procedures with other features like:
- View of every important object in a single place
- Development progress transparency
- Project Linkage to Department standards
- Identification of issues for Work items needing Attention
Traceability and Transparency
Achieve IEC 62304 compliance with Requirements traceability. IBM ELM offers great help with the support of Software Requirement Analysis by tracing software requirements to its parent requirements, while also linking to test cases and test procedures with other features like:
- View of every important object in a single place
- Development progress transparency
- Project Linkage to Department standards
- Identification of issues for Work items needing Attention
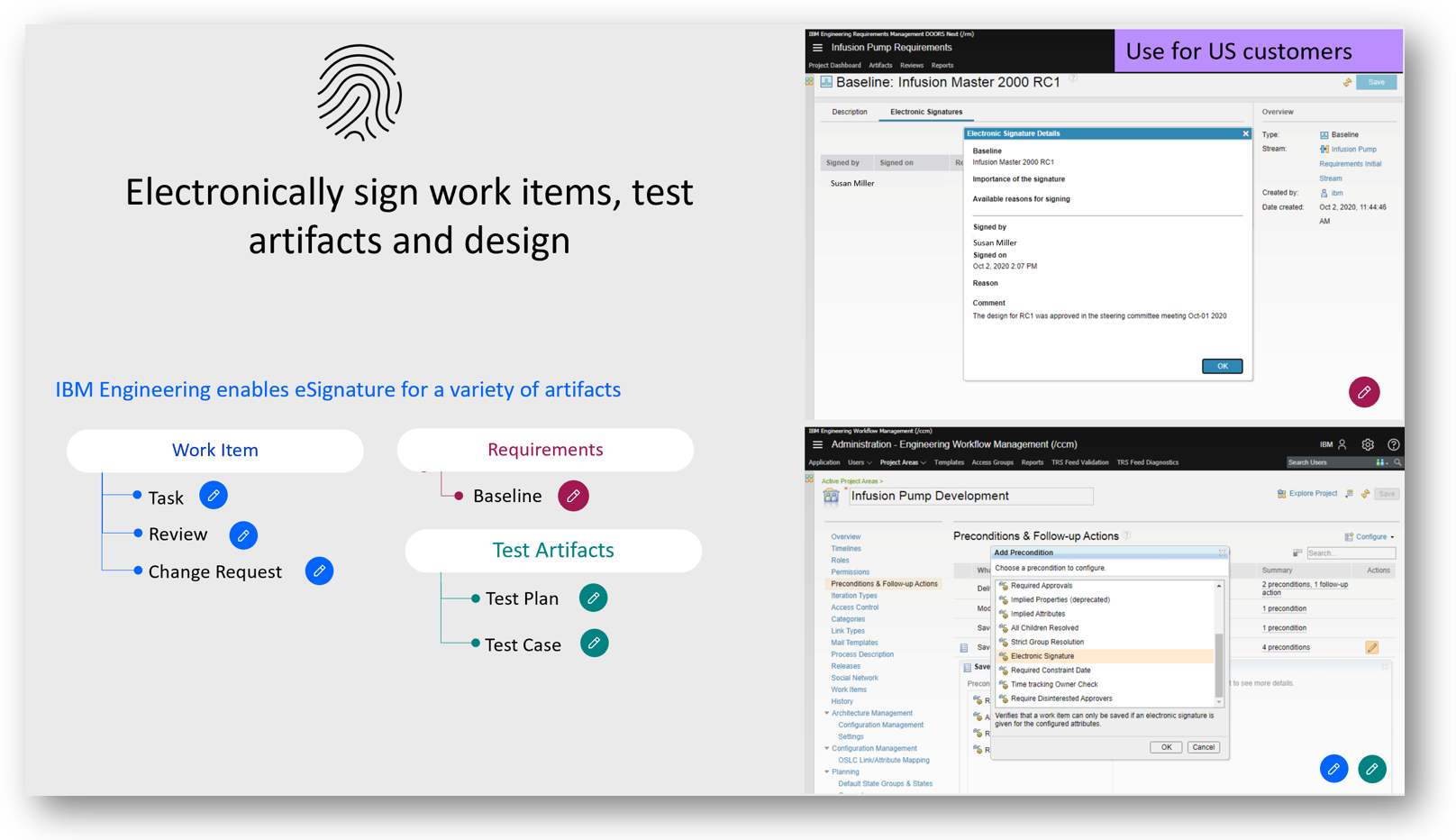
E-Signatures
Sign specific configuration of your specification (baseline) with electronic signatures compliant with CFR Part 11 process from the FDA. The signatures can also be captured, and the information can be printed into a document.
Example:
- Name of the signing Person
- Reason for signing
- Signature date
- Role
- Specific custom text
- Approval comment
Automate Your Validation and Verification
Validation and verification is supported via comprehensive traceability, automated forensic-level accountability, and real-time reporting throughout the lifecycle as well as across projects.
Furthermore, it is supported via review and approval of any work item with an electronic signature, combined with uninterrupted traceability starting at the design phase.
Automate Your Validation and Verification
Validation and verification is supported via comprehensive traceability, automated forensic-level accountability, and real-time reporting throughout the lifecycle as well as across projects.
Furthermore, it is supported via review and approval of any work item with an electronic signature, combined with uninterrupted traceability starting at the design phase.
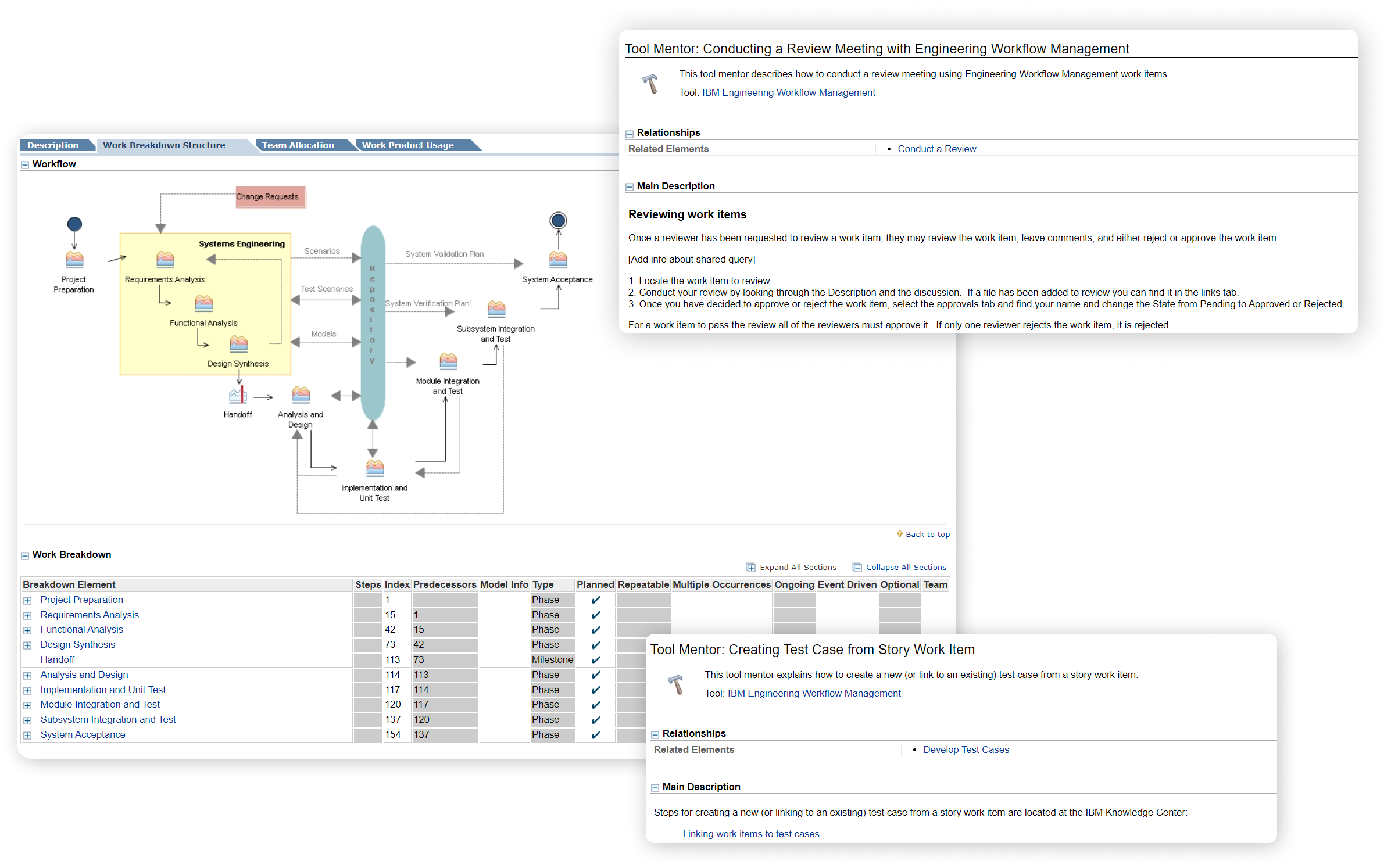
Rapid Product Development
Rapid Product Development of your products are greatly accelerated by IBM Engineering Workflow Management, which acts as the critical link between required and delivered work by enabling teams to manage plans, tasks and project status.
It provides the flexibility to adapt to any process, so companies can adopt faster release cycles and manage dependencies across both small and complex development projects.
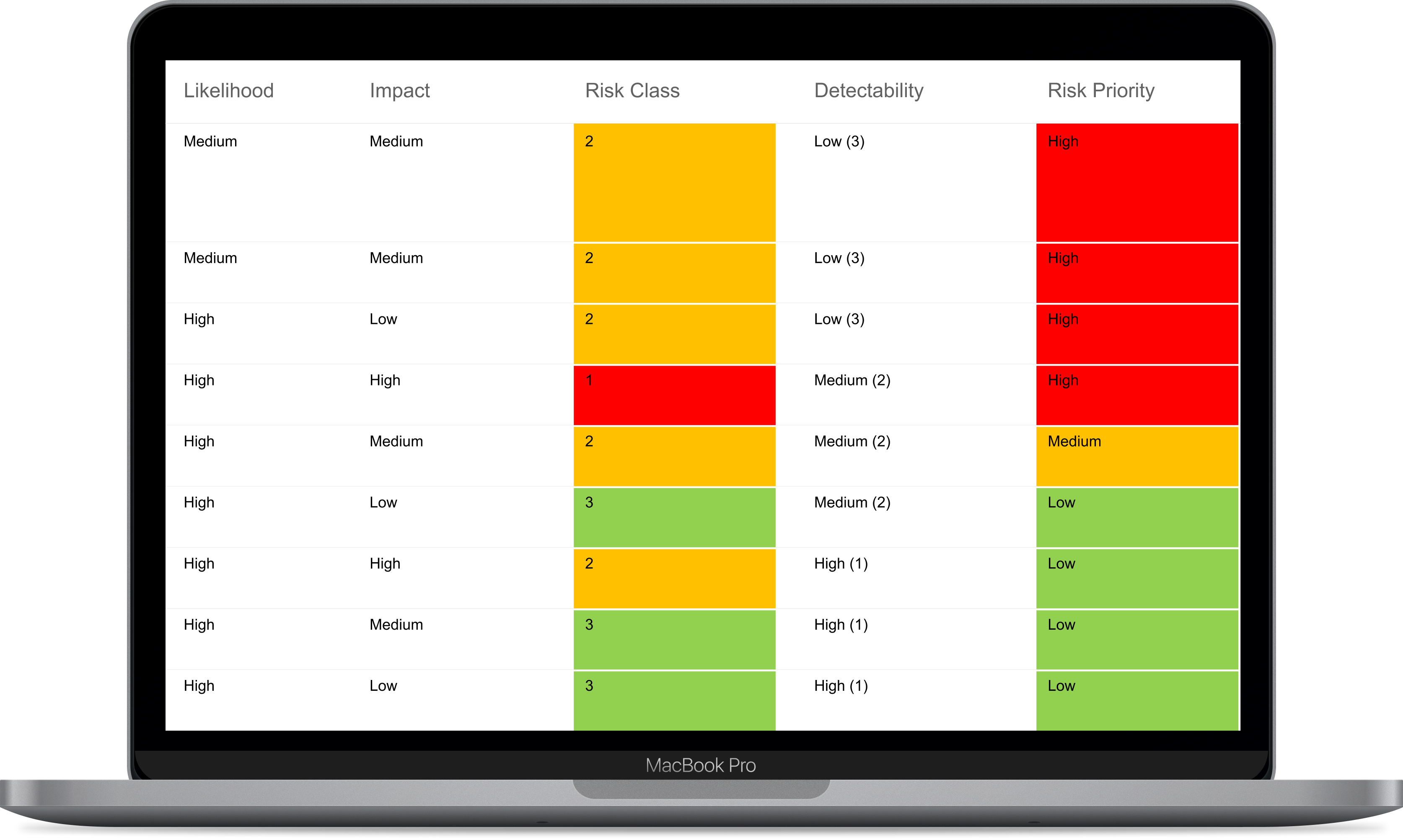
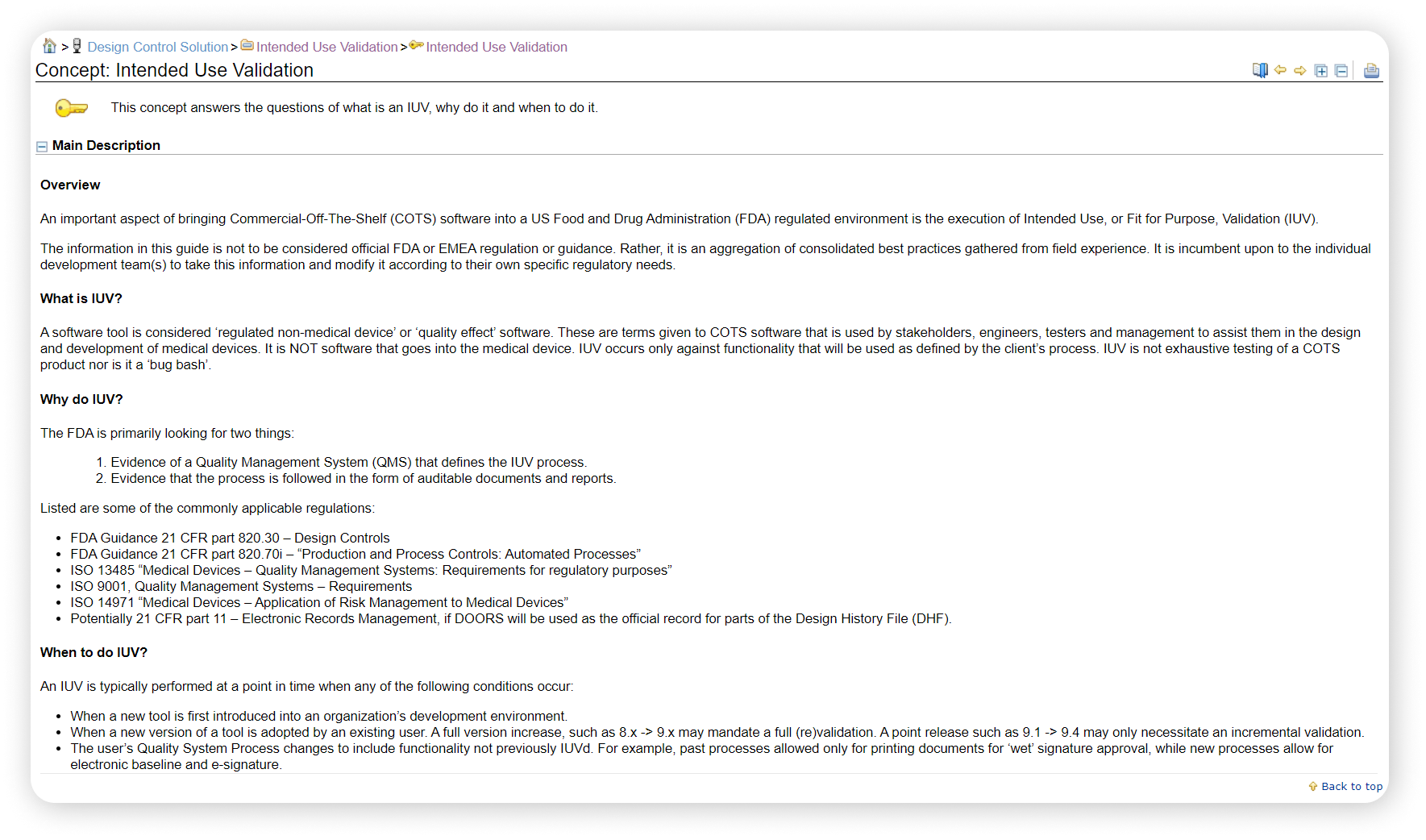
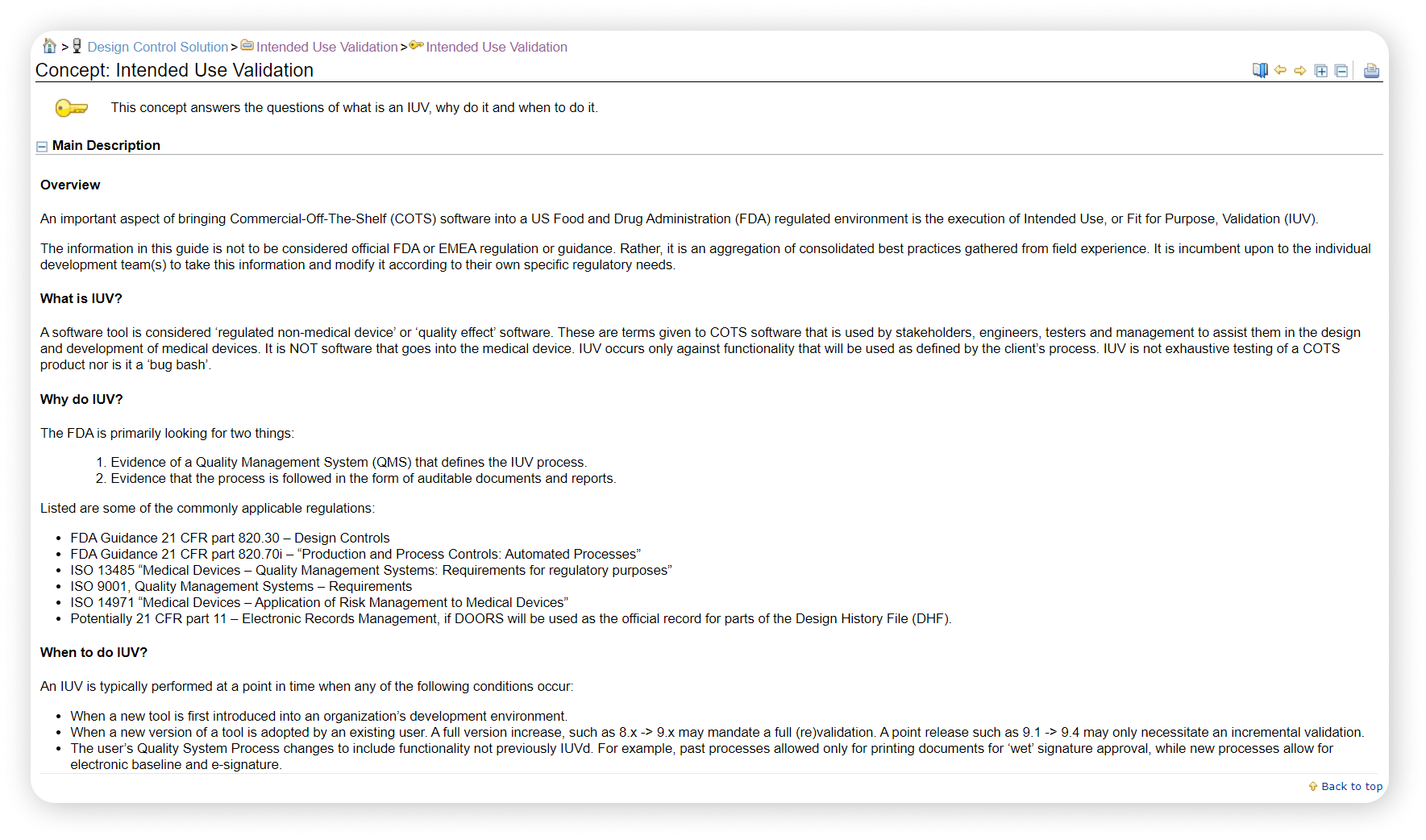
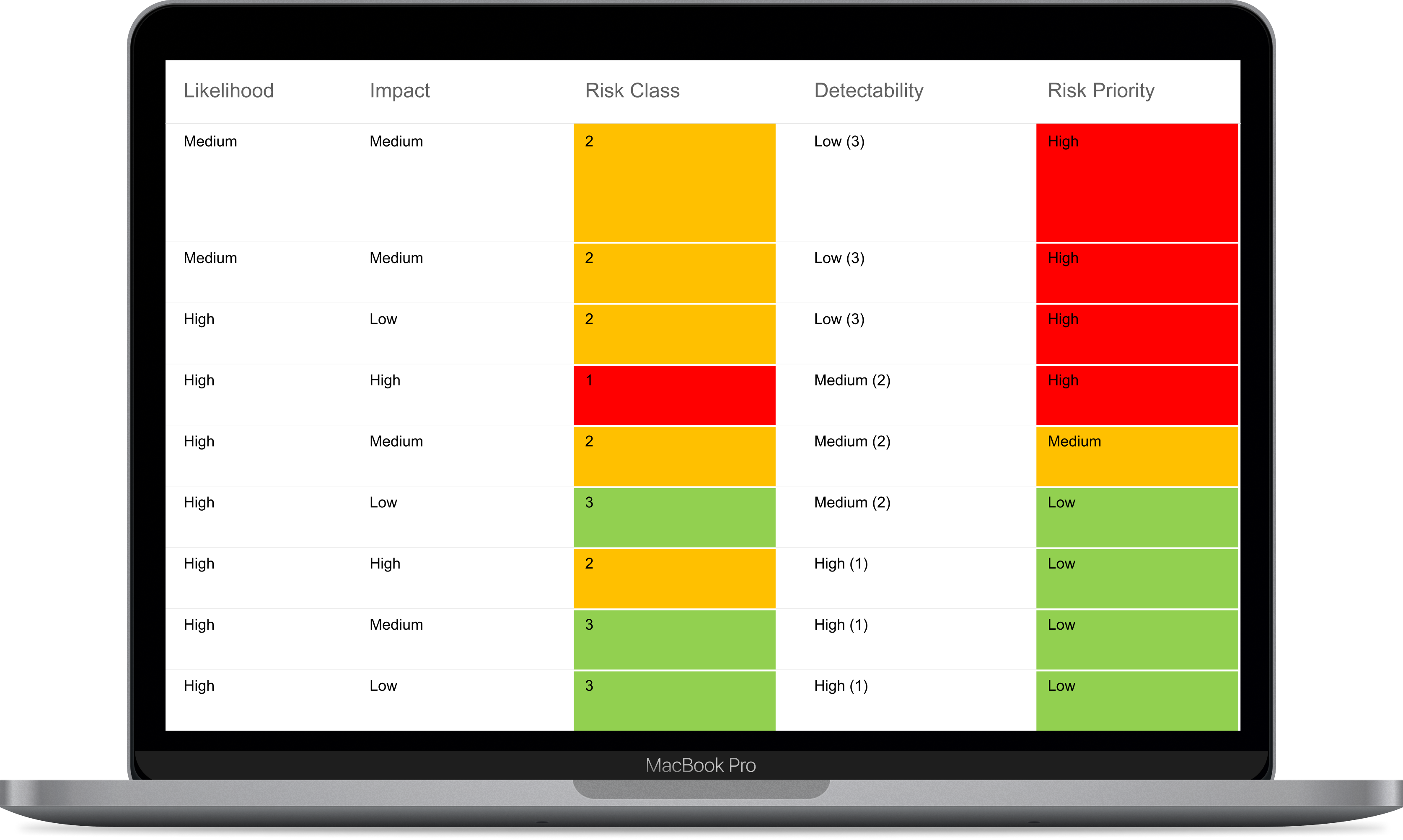
ISO 14971 Risk Mangement for Medical Devices
IBM ELM provides support in compliance for ISO 14971 by providing key features that help you and your team throughout your process of designing Medical Devices such as:
- Collaborative Risk Analysis
- Intended Use Validation
- Configurable KPI Dashboards
- Integrated CAPA System
Read more about our support for ISO 14971 Risk Management for Medical Devices in IBM ELM
ISO 14971 Risk Mangement for Medical Devices
IBM ELM provides support in compliance for ISO 14971 by providing key features that help you and your team throughout your process of designing Medical Devices such as:
- Collaborative Risk Analysis
- Intended Use Validation
- Configurable KPI Dashboards
- Integrated CAPA System
Read more about our support for ISO 14971 Risk Management for Medical Devices in IBM ELM
Success stories of our clients
Whitepapers
Overview of the solution for medical devices and its value add for development teams. Softacus Flyer
Improving development processes using IBM Rational DOORS Requirements Management software in Medical Devices Industry
Accelerate development of products while meeting IEC 61508, IEC 62304, ISO 14971, ISO 13485 and 21 CFR 820.30
Address the FDA identified lack of design controls as one of the major causes of device recalls
How to manage your compliance with IBM Tools in Medical Device Development
Automating product development and compliance processes using IBM Rational software
This paper will explore what IEC 62304 compliance means for manufacturers in some detail, and also describe the larger context of systems and software engineering best practices at work in many of today’s most successful companies.
Drei strategische Imperativen, für effiziente und
erfolgreiche Medizintechnik Entwicklungsprojekte
Improve processes, manage IEC 61508 and IEC 62304 standards, develop quality products
Whitepapers
Overview of the solution for medical devices and its value add for development teams. Softacus Flyer
Improving development processes using IBM Rational DOORS Requirements Management software in Medical Devices Industry
Accelerate development of products while meeting IEC 61508, IEC 62304, ISO 14971, ISO 13485 and 21 CFR 820.30
Address the FDA identified lack of design controls as one of the major causes of device recalls
How to manage your compliance with IBM Tools in Medical Device Development
Automating product development and compliance processes using IBM Rational software
This paper will explore what IEC 62304 compliance means for manufacturers in some detail, and also describe the larger context of systems and software engineering best practices at work in many of today’s most successful companies.
Drei strategische Imperativen, für effiziente und
erfolgreiche Medizintechnik Entwicklungsprojekte
Improve processes, manage IEC 61508 and IEC 62304 standards, develop quality products